Which of the Following Distinguishes Hydrogen Bonds From Covalent Bonds
The atoms of non-metals tend to be. While the three B F covalent bonds are formed due to the sharing of electron pairs resulting from contributions of both boron and fluorine atoms an N B bond is formed due to the donation of a lone pair of electrons from nitrogen into the empty orbitals of boron.
Difference Between Covalent And Hydrogen Bonds Definition Formation Of Bond Examples
Owing to these properties non-metals usually gain electrons when reacting with other compounds forming covalent bonds.
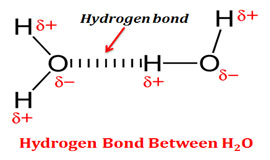
. Non-Metal dopants are carbon nitrogen fluorine sulphur and iodine. Among the non-metals the anionic dopants have a strong influence on the VB. The following are the general properties of non-metals.
Figure 2 distinguishes the covalent bonds from the coordinate covalent bond in NH 3 BF 3.

Difference Between Covalent And Hydrogen Bond Easy Biology Class

Chemical Bonds Anatomy And Physiology I
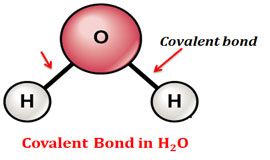
Difference Between Covalent And Hydrogen Bond Easy Biology Class
No comments for "Which of the Following Distinguishes Hydrogen Bonds From Covalent Bonds"
Post a Comment